Research Updates
THRUST 1 - DEHYDROGENATION
Jason Hicks, Thrust 1 Lead
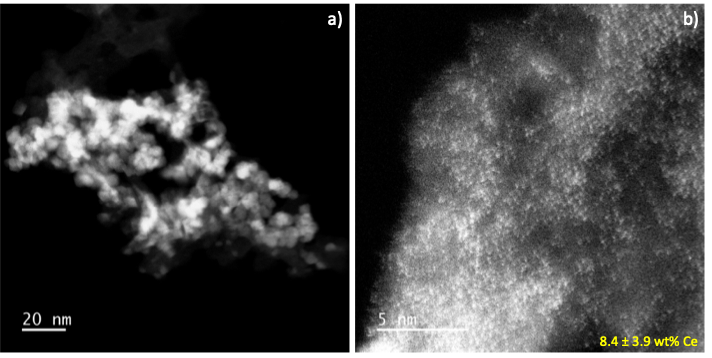
As part of our strategy to develop thermally stable and regenerable catalysts, Thrust 1 researchers have extended our work on ceria supports to a study of ceria supported on alumina. Cerium oxide (ceria) has been shown to be very effective at trapping platinum atoms due to formation of stable surface complexes at step edges, where coordinatively unsaturated cerium cations are present. But ceria loses its effectiveness when heated to high temperatures due to loss of surface area and growth in particle size associated with sintering of the oxide. Being a rare earth, and with limited supplies worldwide, it is important to develop methods to improve the effectiveness of ceria as a catalyst support. In a recent report, Thrust 1 researchers explore the performance for trapping Pt atoms when the ceria is supported on a high surface area alumina carrier.
This helps create a more sustainable catalyst formulation, especially if we can retain the high dispersion of Pt seen on ceria supports. For this work, we studied the atom trapping efficacy of ceria/alumina samples with increasing ceria content (8−50 wt %) and contrasted the behavior with pure ceria. Electron microscopy reveals that when dispersed on alumina, ceria is present in the form of crystalline nanoparticles and isolated cerium ions. These two forms of ceria differ markedly in their ability to trap Pt atoms. Atomically dispersed cerium is present in the form of Ce3+ cations on alumina; however, this form of ceria is not effective for trapping Pt atoms. Our results show that the atom-trapped Pt resides primarily on crystalline ceria nanoparticles. CO oxidation was used as a probe reaction to evaluate the performance of these PtAT/ ceria-alumina catalysts. We conclude that over the range of ceria loadings we investigated, 50% ceria/alumina represents the optimal catalyst support for achieving high surface area and atom trapping efficiency while helping reduce the total ceria content in this catalyst system.
AC-STEM images of 50 wt% ceria/alumina showing a) a high concentration of ceria crystallites uniformly dispersed on the alumina. b) The regions devoid of crystallites show a high concentration of single atoms of Ce (seen as bright dots), and by EDS the support has ~ 8.4 wt% atomically dispersed Ce on alumina.
Hien N. Pham, Andrew DeLaRiva, Eric J. Peterson, Ryan Alcala, Konstantin Khivantsev, János Szanyi, Xiaohong S. Li, Dong Jiang, Weixin Huang, Yipeng Sun, Pascaline Tran, Quan Do, Craig L. DiMaggio, Yong Wang*, and Abhaya K. Datye*, ACS Sustainable Chem. Eng. 2022, 10, 23, 7603-7612, DOI: 10.1021/acssuschemeng.2c01380
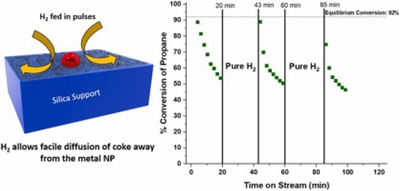
As noted above, catalyst stability, resistance to deactivation, and regeneration remain a challenge for high-temperature reaction processes in Thrust 1. For Pt alloys used in propane dehydrogenation (PDH), the primary pathways of catalyst deactivation include coke formation and metal nanoparticle sintering over time. Recent work shows that silica-supported catalysts provide excellent selectivity for this reaction, but the regenerability of silica-supported catalysts has not been established. Recently, Thrust 1 researchers studied a series of Pt alloys, including PtMn, PtZn, and PtSn, for the PDH reaction at 550 °C and 600 °C, and we subjected the catalysts to regeneration over multiple cycles. While oxidation in air restores the reactivity completely with minimal catalyst sintering, it is surprising to find that these catalysts can also be regenerated in pure hydrogen. In this publication, we explore the types of coke formed on these catalysts using in situ temperature programmed oxidation (TPO). Two types of coke are found: one on the metallic NP surface, and a second on the silica support. Our work shows that treatment in hydrogen causes redistribution of the coke between the metal and support, which can restore most catalytic activity lost during a reaction run. Periodic introduction of H2 during a reaction cycle may constitute an unexplored strategy for extending the lifetime of PDH catalysts.
Alcala, R.; Dean, D. P.; Chavan, I.; Chang, C.-W.; Burnside, B.; Pham, H. N.; Peterson, E.; Miller, J. T.; Datye, A. K., Strategies for regeneration of Pt-alloy catalysts supported on silica for propane dehydrogenation. Applied Catalysis A: General 2023, 658, 119157. https://doi.org/10.1016/j.apcata.2023.119157
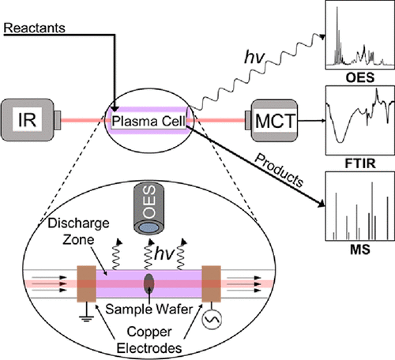 Plasma-surface coupling has emerged as a promising approach for CISTAR Thrust 1 researchers to perform chemical transformations under mild conditions that are otherwise difficult thermally. However, a few examples of inexpensive and accessible in situ/operando techniques exist for observing plasma-solid interactions, which has prevented a thorough understanding of underlying surface mechanisms. In this manuscript, Thrust 1 researchers provide a simple and adaptable design for a dielectric barrier discharge (DBD) plasma cell capable of interfacing with Fourier transform infrared spectroscopy (FTIR), optical emission spectroscopy (OES), and mass spectrometry (MS) to simultaneously characterize the surface, the plasma phase, and the gas phase, respectively. The system was demonstrated using two example applications: (1) plasma oxidation of primary amine functionalized SBA-15 and (2) catalytic low-temperature nitrogen oxidation. The results from application (1) provided direct evidence of a 1% O2/He plasma interacting with the aminosilica surface by selective oxidation of the amino groups to nitro groups without altering the alkyl tether. Application (2) was used to detect the evolution of NOX species bound to both platinum and silica surfaces under plasma stimulation. Together, the experimental results showcase the breadth of possible applications for this device and confirm its potential as an essential tool for conducting research on plasma-surface coupling.
Plasma-surface coupling has emerged as a promising approach for CISTAR Thrust 1 researchers to perform chemical transformations under mild conditions that are otherwise difficult thermally. However, a few examples of inexpensive and accessible in situ/operando techniques exist for observing plasma-solid interactions, which has prevented a thorough understanding of underlying surface mechanisms. In this manuscript, Thrust 1 researchers provide a simple and adaptable design for a dielectric barrier discharge (DBD) plasma cell capable of interfacing with Fourier transform infrared spectroscopy (FTIR), optical emission spectroscopy (OES), and mass spectrometry (MS) to simultaneously characterize the surface, the plasma phase, and the gas phase, respectively. The system was demonstrated using two example applications: (1) plasma oxidation of primary amine functionalized SBA-15 and (2) catalytic low-temperature nitrogen oxidation. The results from application (1) provided direct evidence of a 1% O2/He plasma interacting with the aminosilica surface by selective oxidation of the amino groups to nitro groups without altering the alkyl tether. Application (2) was used to detect the evolution of NOX species bound to both platinum and silica surfaces under plasma stimulation. Together, the experimental results showcase the breadth of possible applications for this device and confirm its potential as an essential tool for conducting research on plasma-surface coupling.
Clarke, R. J.; Hicks, J. C., Interrogation of the Plasma-Catalyst Interface via In Situ/Operando Transmission Infrared Spectroscopy. ACS Engineering Au 2022, 2 (6), 535-546. https://doi.org/10.1021/acsengineeringau.2c00026
THRUST 2 - OLIGOMERIZATION
Rajamani Gounder, Thrust 2 Lead
T2P1: Development of Stable and Selective Zeolite Catalysts for Olefin Oligomerization
JACS Au, (2022); doi: 10.1021/jacsau.2c00462
ACS Catalysis, (2023); doi: 10.1021/acscatal.2c05184
Elizabeth E. Bickel, Songhyun Lee, Evan Sowinski, Rajamani Gounder
Brønsted acid zeolites catalyze olefin oligomerization, a reaction relevant for upgrading light olefins to heavier molecules useful as liquid fuels. Our recent publications discuss efforts to synthesize MFI and other zeolite topologies with independently varied crystallite size and acid site density, and to determine their effects on oligomerization rates, product selectivities, and time-on-stream stability. We provide evidence that heavier alkene oligomers gradually accumulate within micropores of medium-pore (ten-membered ring, 10-MR) zeolites during oligomerization catalysis, and that the composition of these occluded hydrocarbons changes in response to reaction conditions and material properties, imposing intracrystalline diffusion barriers that influence rates of molecular transport. Measured rates and selectivities of olefin oligomerization reflect the combined influences of the kinetic properties of active sites and diffusional constraints imposed by occluded intrapore hydrocarbons, leading to complex dependences of rates and selectivity on alkene pressure and material properties among 10-MR zeolite catalysts (Figure 1). Armed with this fundamental knowledge, new CISTAR zeolite catalysts are being developed and patented that have improved transport properties leading to higher reaction rates and formation of liquid products in higher yields and with stable time-on-stream compositions.
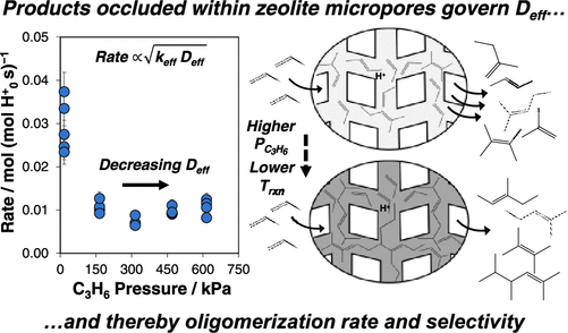
Figure 1. Hydrocarbon products occlude within MFI zeolite pores during oligomerization catalysis and impose diffusional restrictions that regulate observed reaction rates.






