Research Update
Jump to Thrust Section:
THRUST ONE
Dehydrogenation
Jason Hicks, University of Notre Dame
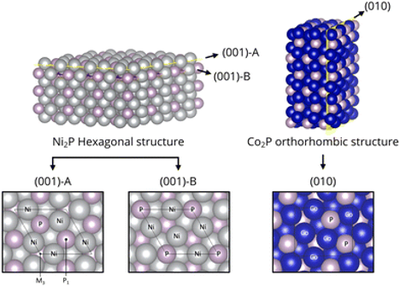 Thrust 1 researchers have identified metal phosphides as promising catalysts for hydrocarbon transformations, but computational screening is complicated by their diverse structures and compositions. To disentangle structural from compositional contributions, here we explore the metal-rich M2P (M = Fe, Co, Ni, Cu, Mo, Ru, Rh, Pd, Ag, Pt) series in hexagonal and orthorhombic structures that are common to a subset of these materials, using supercell density functional theory (DFT). To understand the contribution of metal choice to the utility for catalytic ethane dehydrogenation (EDH), we compute and compare the adsorption of key EDH intermediates across low-index surface terminations. These materials expose both metal and phosphide sites. Calculations show that binding energies at metal sites correlate with the bulk metals, with P incorporation either enhancing or suppressing binding. Phosphide sites compete with metal sites for adsorbates and tend to suppress overactivation by destabilizing highly dehydrogenated species engaging in C–H bond breaking. Results are generally insensitive to bulk structure and surface facet. Results suggest metal-rich Pd phosphides have favorable adsorption characteristics for catalytic dehydrogenation, consistent with recent observations.
Thrust 1 researchers have identified metal phosphides as promising catalysts for hydrocarbon transformations, but computational screening is complicated by their diverse structures and compositions. To disentangle structural from compositional contributions, here we explore the metal-rich M2P (M = Fe, Co, Ni, Cu, Mo, Ru, Rh, Pd, Ag, Pt) series in hexagonal and orthorhombic structures that are common to a subset of these materials, using supercell density functional theory (DFT). To understand the contribution of metal choice to the utility for catalytic ethane dehydrogenation (EDH), we compute and compare the adsorption of key EDH intermediates across low-index surface terminations. These materials expose both metal and phosphide sites. Calculations show that binding energies at metal sites correlate with the bulk metals, with P incorporation either enhancing or suppressing binding. Phosphide sites compete with metal sites for adsorbates and tend to suppress overactivation by destabilizing highly dehydrogenated species engaging in C–H bond breaking. Results are generally insensitive to bulk structure and surface facet. Results suggest metal-rich Pd phosphides have favorable adsorption characteristics for catalytic dehydrogenation, consistent with recent observations.
- Jeonghyun Ko, Hanyu Ma, and William F. Schneider*, Computational screen of M2P metal phosphides for catalytic ethane dehydrogenation, Catal. Sci. Technol., 2022, Advance Article, DOI: 10.1039/D2CY00602B
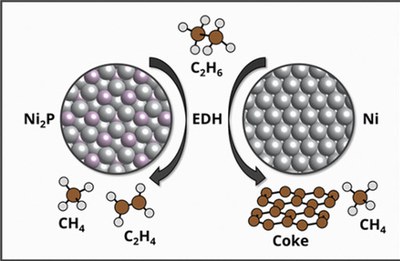 In a separate study, Thrust 1 researchers used supercell density functional theory (DFT) calculations to create and exercise complete ethane dehydrogenation (EDH) reaction networks over Ni(111) and Ni2P(001). EDH intermediates are predicted to be more weakly bound to Ni2P than to Ni, with the differences being the greatest for deeply dehydrogenated intermediates. Both C–H and C–C bond cleavage activation energies are generally greater on Ni2P than Ni. The implications of these differences are explored through microkinetic models that incorporate a pathway for carbon growth. Ethylene formation rates and selectivities are predicted to be greater over Ni2P than Ni across a wide range of temperatures. Ni is predicted to be more susceptible to coke generation. Ethane activation exerts the greatest degree of rate control on both materials; in contrast, the competition between ethylene desorption and dehydrogenation controls selectivity on Ni, while no single step dominates selectivity on Ni2P. Results are consistent with previously reported observations, highlight the distinct and active contributions of phosphorus to phosphide surface chemistry, and highlight the role of coking pathways on predicted kinetics.
In a separate study, Thrust 1 researchers used supercell density functional theory (DFT) calculations to create and exercise complete ethane dehydrogenation (EDH) reaction networks over Ni(111) and Ni2P(001). EDH intermediates are predicted to be more weakly bound to Ni2P than to Ni, with the differences being the greatest for deeply dehydrogenated intermediates. Both C–H and C–C bond cleavage activation energies are generally greater on Ni2P than Ni. The implications of these differences are explored through microkinetic models that incorporate a pathway for carbon growth. Ethylene formation rates and selectivities are predicted to be greater over Ni2P than Ni across a wide range of temperatures. Ni is predicted to be more susceptible to coke generation. Ethane activation exerts the greatest degree of rate control on both materials; in contrast, the competition between ethylene desorption and dehydrogenation controls selectivity on Ni, while no single step dominates selectivity on Ni2P. Results are consistent with previously reported observations, highlight the distinct and active contributions of phosphorus to phosphide surface chemistry, and highlight the role of coking pathways on predicted kinetics.
Jeonghyun Ko, Hanyu Ma, and William F. Schneider*, Kinetic Origins of High Selectivity of Metal Phosphides for Ethane Dehydrogenation, Ind. Eng. Chem. Res., 2022, https://doi.org/10.1021/acs.iecr.2c02044
THRUST 2
Oligomerization
Jeff Miller, Purdue University
T2P5: Insights into the Chemistry of the Homogeneous Thermal Oligomerization of Olefins to Liquid-Fuel Range Hydrocarbons
Ind. & Eng. Chem. Res, (2022); doi: 10.1021/acs.iecr.2c02172
Matthew A. Conrad, Alexander Shaw, Grant Marsden, Linda J. Broadbelt, Jeffrey T. Miller
Thermal, non-catalytic conversion of light olefins (C2= - C4=) was originally utilized in the production of motor fuels at several U.S. refineries in the 1920-30’s. However, the resulting fuels had relatively low-octane numbers and required harsh operating conditions (T > 450 oC, P > 50 bar), ultimately leading to its succession by solid acid catalytic processes. Despite the early utilization of the thermal reaction, relatively little is known about the reaction products, kinetics, and initiation pathway under liquid-producing conditions. In this study, thermal ethylene oligomerization was investigated near the industrial operating conditions, i.e, at temperatures between 300 and 500 oC and ethylene pressures from 1.5 to 43.5 bar. Non-oligomer products such as propylene and/or higher odd carbon products were significant at all reaction temperatures, pressures, and reaction extents. Methane and ethane were minor products (< 1 % each), even at ethylene conversions as high as 74 %.
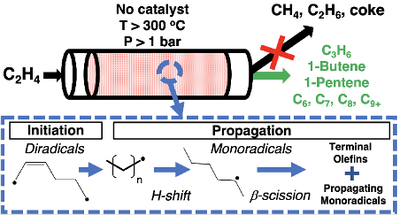
Figure: Above 300°C and especially at high pressures, ethylene reacts thermally to produce higher molecular weight olefins from propylene to C9+
The isomer distributions revealed a preference for linear, terminal C4, and C5. The reaction order was found to be 2nd order with a temperature-dependent overall activation energy ranging from 39.4 to 58.3 kcal mol-1. Four bimolecular initiation reaction steps for ethylene were calculated using DFT. Of these, simple H-transfer to yield vinyl and ethyl radicals was found to have a free energy activation energy barrier higher (about 10 kcal mol-1) than the other three initiation steps forming either cyclobutane, 1-butene, or tetramethylene. The importance of diradical species in generating free radicals during a two-phase initiation process was proposed in Figure. The reaction chemistry for ethylene, which has only strong, vinyl C-H bonds, starkly contrasted with propylene, which possesses weaker allylic C-H bonds and showed a preference for dimeric C6 products over C2-C8 non-oligomers. The resulting C4 and C5 non-oligomers from propylene contained more iso-olefins compared to linear C4 and C5
THRUST 3
C1 Activation
Tobin Marks, Northwestern University
Thrust 3 project teams have made significant progress in three main directions related to the conversions of methane and related light alkanes to olefins. Within T3P3, led by Tobin Marks, developed and characterized a supported vanadium catalyst that provides exceptional propane to propene yields using S2 as the oxidant. Within T3P4, led by Fernando Garzon, has realized very stable doped barium niobate-based electrocatalysts for the oxidative electrochemical coupling of methane to ethylene. Within T3P5, led by Jeff Miller, with important input from Abhaya Datye has carried out incisive mechanistic studies of the conversion of propane to higher molecular weight hydrocarbons over a bifunctional PtZn/SiO2 + ZSM5 catalyst. Details of each project’s contributions are provided below.
T3P3 Research Highlight (Marks). In Catalytic Oxidative Dehydrogenation, Why does Propane More Readily Undergo Overoxidation with O2 than with S2?
Thrust 3 has continued to work on the development of new and active/selective catalysts for light alkane conversion to olefins. In addition, Thrust 3 researchers have continued investigating supported vanadium catalysts for oxidative coupling of propane using sulfur as an alternative oxidant. The new findings suggest that propylene is more favorably desorbed from the sulfided surface than the oxidized surface of V/MxOy catalysts. This result, as well as the fact that propane over-oxidation is less thermodynamically favorable using S2 oxidant, synergistically led to a higher propylene selectivity compared to the oxygen counterpart. Theory collaborations with the Jeff Greeley group at Purdue are currently underway to further explore this hypothesis as is EXAFS/XANES work with the Miller group at Purdue.
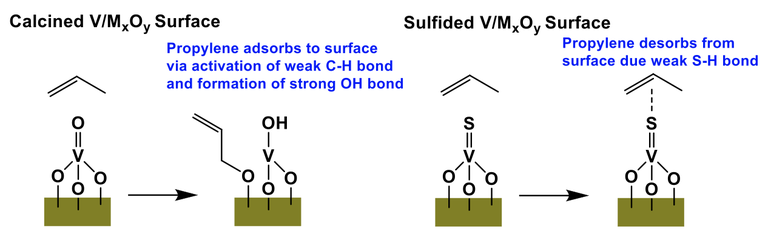
Figure: Proposed interaction of the calcined and sulfided V/MxOy surfaces with propylene in catalytic propane to propylene conversion.
- Arinaga, A.M.; Alayoglu; S.; Zheng, D.; Marks, T.J.; Supported Vanadium Catalysts for Selective Sulfur-Oxidative Dehydrogenation of Propane, ChemCatChem, 2021, 13, 1-7. DOI:10.1002/cctc.202100922
- Arinaga, A.M.; Zhang, X.; Dean, D. A.; Biswas, A.; Miller, J.T.; Greeley, J.; Marks, T.J., Origin of Rate, Selectivity, and 41% Yield Over Supported Vanadium Catalyst-Mediated Sulfur-Oxidative Propane Dehydrogenation, 2022, manuscript in preparation
T3P4 Research Highlight (Garzon). Highly Stable Doped Barium Niobate Based Electrocatalysts for Effective Electrochemical Coupling of Methane to Ethylene
Thrust 3 has continued the development of electrocatalyst materials for the efficient conversion of methane to ethylene in an electrochemical setup. We developed a Fe and Mg co-doped barium niobate perovskites that showed excellent chemical stability in CH4-rich environments up to 925 °C. Temperature-programmed reaction measurements on these catalysts showed methane activation properties from 600 °C with a maximum conversion of 12.5% and selectivity of 50.3% at 800 °C. The electrical conductivity of this perovskite reached about 17 mS cm-1 at 900°C (Figure 1a). Electrochemical Oxidative Coupling of Methane (E-OCM) measurements indicated an ethylene production rate of 277 mmol cm-2 h-1 with a faradaic efficiency of 20% at 1 V. Cyclic voltammetry measurements indicated a durable operation for six continuous days without any drop in peak intensity or product distribution (Figure 1b and 1c). X-ray Photoelectron spectroscopy (XPS) measurements indicate a significant Nb valency reorganization from Nb4+ to Nb5+ that could be attributed as the reason for its chemical stability.
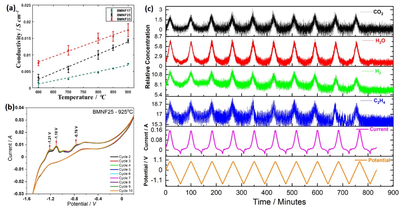
Figure: Conductivity data obtained for the BMNF perovskites with different Fe content in the temperature range of 600 to 900°C, (b) Cyclic voltammetry curves obtained for BMNF25 under CH4 cathode environment, and (c) Current and potential curves along with observed product stream as a function of time during CV measurements.
- Denoyer, L.; Benavidez, A.; Garzon, F.; Ramaiyan, K. Highly Stable Doped Barium Niobate Based Electrocatalysts for Effective Electrochemical Coupling of Methane to Ethylene, Advanced Materials Interfaces, Accepted August 2022.
T3P5 Research Highlight (Miller). Dehydroaromatization Pathway of Propane on PtZn/SiO2 + ZSM5 Bifunctional Catalyst
The Cyclar process was previously developed to convert propane and butane into aromatics using gallium-loaded ZSM-5 catalysts (Ga/ZSM-5). However, the BTX (benzene, toluene, xylenes) yield is limited by light gas formation, primarily methane and ethane. The relative rates and selectivity for propane conversion on two catalytic components, gallium (Ga/Al2O3) and acid ZSM-5 (H-ZSM-5) suggest that light gas was produced by propane monomolecular cracking on ZSM-5 due to the imbalance of alkane dehydrogenation and olefin conversion rates on two catalytic functions. A PtZn alloy catalyst, which has >99% propene selectivity and 100 times higher rate than Ga, was used for the dehydrogenation function. The bifunctional PtZn/SiO2 + H-ZSM-5 catalyst has high yields of aromatics with low selectivity to methane (<5%) at ~70% propane conversion. The results suggest light gas yield can be minimized by utilizing the PtZn alloy and lowering the monomolecular cracking rate by ZSM-5 giving approximately 80% yield at full recycle.
Due to the much higher dehydrogenation rate of PtZn alloy, the temperature effect on propane dehydroaromatization pathways on the PtZn/SiO 2 +ZSM-5 bifunctional catalysts is investigated. As discussed above, high temperature (550℃), high dehydrogenation rates and lower monomolecular cracking rates are required to minimize methane formation, leading to primarily propene and BTX (benzene, toluene, and xylenes). At mid temperature (400-450℃), the product selectivity is to gasoline-blending hydrocarbons such as butanes, C 5 + hydrocarbons, toluene, and xylenes at 15-25% propane conversions. At low temperature (350℃), ~25% propane conversion is achieved and has high selectivity (~60%) to butanes, however, the propane conversion rates are likely too low to be practical. While methane formation by monomolecular cracking limits liquid yields at the high reaction temperature, at mid and low temperatures, co-produced hydrogen saturates light olefins to undesired ethane, which becomes a major yield loss reaction.
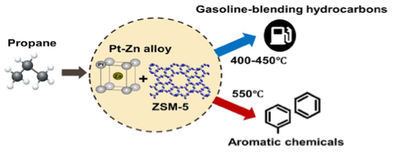
Figure: The temperature dependence on product selectivity for bifunctional conversion of propane to higher molecular weight hydrocarbons leads to different products.
- C.-W. Chang, H.N. Pham, R. Alcala, A.K. Datye, J.T. Miller of:
- ACS Sustainable Chem. & Eng., 10(1), 394-409 (2022), DOI:10.1021/acssuschemeng.1c06579
- Appl. Catal. A, Gen., 643, 118753 (2022): DOI: 10.1016/j.apcata.2022.118753.
THRUST 6
Membrane Separations
Ruilan Guo, University of Notre Dame
Thrust 6 researchers continue researching high-performance membrane materials to meet the demanding gas separation needs in CISTAR, including H2/C1-3, C1/C2+, and olefin/paraffin separations. In the project of developing olefin/paraffin separation membranes led by Joan Brennecke and Benny Freeman research groups at UTA, CISTAR researchers have successfully translated the discoveries from the supported ionic liquid membranes (SILMs) using anopore disc supports (Angew. Chem. Int. Ed. 2022, e202202895) to easily scalable polymer supports. These polyelectrolyte membranes containing Ag+ in crosslinked PEO matrix have as good or better permeabilities, selectivities, and hydrogen stability as the anopore disc SILMs. An invention disclosure entitled “Selective and Hydrogen-stable Solid Polymer Electrolyte Membranes for Olefin-Paraffin Separation” was filed and the results were also published in Journal of Membrane Science, 2022, 647, 120300. The development of microporous iptycene-containing polybenzoxazole (PBO) membranes for H2/HC separations (led by Ruilan Guo's group at Notre Dame) has advanced to test the membranes under realistic conditions using the Advanced Membrane Technology Module or high-temperature gas permeation cell. Thermally rearranged pentiptycene-PBO (TR-PPBO) membranes (Chem. Mater. 2022, 34, 2730−2742) showed similarly high H2/CH4 separation performance in the mixed-gas permeation tests outperforming the 2008 upper bound. The crosslinked PPBO membranes (Chem. Mater. 2022, in revision), when tested at elevated temperatures (50-180°C) using a high-temperature pure-gas permeation cell, maintained high separation performance exceeding the 2008 upper bound for H2/CH4 separation (Figure 1).
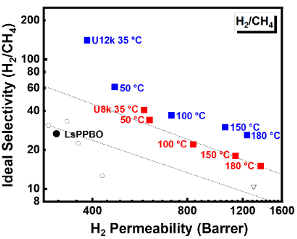
Figure: High-temperature H2/CH4 separation performance of crosslinked PPBO membranes.
Another highlight in Thrust 6 research is the development of greener membrane manufacturing strategies using alternative bio-derived solvents led by Freeman group at UTA. The new strategies were successfully demonstrated on commercial polyimide and polysulfone membranes with well-controlled porous support structures (Journal of Membrane Science, 2022, 644, 120173). Work is undergoing to prepare defect-free asymmetric membranes from a CISTAR polymer membrane material developed by Guo group at UND (Figure 2).
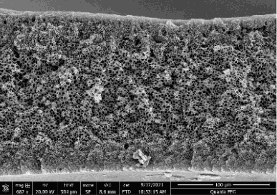
Figure: Defect-free asymmetric membranes prepared from a CISTAR polymer material.






